top of page
LATEST NEWS
FDA Launches New Pathway to Fast-Track Treatments for Ultra-Rare Diseases
The FDA unveils a “plausible mechanism pathway” to speed approval of innovative therapies for ultra-rare diseases, paving the way for personalized and gene-based treatments.
Feb 25


Stem Cell Breakthrough Lets Paralyzed Patient Stand Again
Japanese researchers restored movement in a paralyzed man using reprogrammed stem cells injected into his spinal-cord injury. The treatment, tested in four patients, helped one man stand independently and another regain limb movement, offering early evidence that iPS cell therapies may support neural repair and future paralysis treatments.
Dec 3, 2025


Scientists Cure Type 1 Diabetes in Mice Using a Gentle Immune System “Reset”
Stanford Medicine researchers cured Type 1 diabetes in mice using a gentle immune-reset approach that combines donor blood stem cells and pancreatic islet transplants. The method prevented autoimmunity, required no immunosuppressive drugs, and may pave the way for future treatments for diabetes and other autoimmune diseases.
Dec 2, 2025


LEARN MORE

GENE TECH TIMES
The Future Has Arrived


Capricor's Shares Drop After FDA Rejects Deramiocel Cell Therapy for Heart Disease in DMD
Capricor Therapeutics experienced a major setback after the FDA rejected its cell therapy, Deramiocel, for treating heart disease in Duchenne muscular dystrophy (DMD) patients. The decision led to a 30% drop in Capricor’s stock and highlighted the need for more efficacy data. Deramiocel, which uses cardiac-derived stem cells, aims to regenerate heart tissue and slow cardiomyopathy progression in DMD. Capricor plans to resubmit its application with additional clinical data lat
Jul 16, 2025


FDA Rejects Ultragenyx's Gene Therapy for Rare Disease - Sanfilippo Syndrome
Ultragenyx has received an FDA rejection for its experimental gene therapy targeting Sanfilippo syndrome, a rare and fatal pediatric neurodegenerative disorder. The FDA cited concerns over efficacy data, delaying approval for the treatment. The decision is a major setback for families awaiting a one-time gene therapy option to address the root cause of this rare disease. Ultragenyx plans to work with regulators to resolve the issues and continue advancing its gene therapy pip
Jul 16, 2025


Biogen’s Salanersen SMA Treatment Shows Promise After Gene Therapy
Biogen’s investigational drug, Salanersen, shows strong potential as a once-yearly treatment for spinal muscular atrophy (SMA), even after gene therapies like Zolgensma. Early trial results reveal slowed neurodegeneration and improved motor milestones in children. With reduced neurofilament levels and notable functional gains, Salanersen is heading directly into Phase 3 trials, signaling a possible breakthrough in SMA care and a promising alternative to existing therapies lik
Jul 1, 2025


Sarepta Pauses Elevidys Gene Therapy Trial After Second Death
Sarepta Therapeutics has paused its Duchenne muscular dystrophy (DMD) gene therapy, Elevidys, after a second patient death due to acute liver failure. The pause affects ongoing trials and therapy shipments, raising safety concerns—especially for non-ambulatory patients. Approved under the FDA’s accelerated pathway, Elevidys aimed to slow DMD progression, but recent events have sparked debate over gene therapy risks, regulatory oversight, and treatment decisions for affected f
Jul 1, 2025
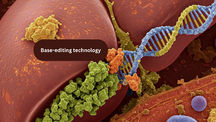
Base Editor Gene Therapy for CPS1 Deficiency Treats Baby’s Rare Disorder
In a historic first, doctors used base editor gene therapy to treat a baby with CPS1 deficiency, a rare genetic liver disorder. The personalized treatment—delivered using lipid nanoparticles—led to improved ammonia levels and reduced medication needs. Unlike traditional CRISPR, base editing made precise DNA changes without cutting, offering a safer, more targeted option. This breakthrough marks a major step forward in treating rare diseases with gene-editing technology.
Jul 1, 2025
bottom of page

